Ref: Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. J. Am. Chem. Soc. 2013, 135(1), 118-121.
Previous Analysis: Just Like Cooking: Post 1, Post 2, author response
Experimenters: Three - Matt Katcher (NJ), Organometallica (IL), B.R.S.M. (United Kingdom)
Recommendation: Moderately reproducible - yields much lower than anticipated
 Trial 1 - Matt
Trial 1 - MattScale: 1 mmol
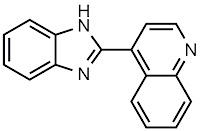
Reaction (table, #): Table 2, entry 2
Reagents: Fe was an old Fisher bottle (40 mesh)
Observations: Tried to keep reaction air-free by setting up with a septum over a pressure tube, but I was not extremely rigorous. Fe stuck to stir bar at end of reaction
Purification: Not attempted
Yield - Published: 83%
Actual: <5% by crude NMR
Advice: I still think the reaction might work, but it may not be a simple "dump and stir". If I did it again, I would run it in a Schlenk tube to minimize exposure to air.
Spectra / Photos:
 |
| Reaction setup |
 |
| LC/MS - Target ion: 246 m/z |
 |
| TLC |
 |
| Crude NMR |
 Trial 2 - B.R.S.M.
Trial 2 - B.R.S.M.Scale: 5 mmol
Reaction (table, #): Table 2, entry 1
Reagents: 2-Nitroaniline (Aldrich 98%; of indeterminate age, because I borrowed it), sulfur (Fisher 'lab reagent' grade, fine powder, age unknown), iron (Fisher '97% reduced by hydrogen grade' [no mesh given]), 4-picoline (10 mmol; Aldrich 98%; 1 year old)
Observations: Used 10-mL Schlenk tube that had been dried under vacuum using a heat-gun. The flask was evacuated and back-filled with argon three times. Dark-coloured suspension, agitated at maximum speed by magnetic stir bar.
Purification: Silica gel. Elution with 100:0 - 95:5 CH2Cl2:MeOH gave the product as a beige foam, still containing some impurities (Rf 0.45 in 96:4). Second attempt: Recrystallisation from toluene-hexane (ca 3:1) gave small yellow-golden brown crystals that were dried overnight in vacuo.
Yield - Published: 90%
Actual: before recryst 366 mg (37%); after 241 mg (24%).
Advice: A crude sample of reaction mixture removed at 24 h indicated around 5:1 aniline:product, but this was probably not reliable due to the low solubility of the product in chloroform. The reaction was quite messy, the product streaky and the column non-trivial.
Spectra / Photos:
 |
| 400 MHz NMR, after rexstal |
 |
| Final product |
 Trial 3 - Organometallica
Trial 3 - OrganometallicaScale: 5 mmol
Reaction (table, #): Table 2, entry 3
Reagents: Fe (Aldrich, >99% powder, fine), S (Aldrich, >99.998%, recrystallized)
Observations: Reaction run in inert-atmosphere glovebox in Schlenk bomb. Noted "internet accounts" (trials 1 & 2) before beginning this reaction.
Purification: Pushed through silica pad with DCM, 10%MeOH / DCM. Distilled on bulb-to-bulb apparatus to remove excess picoline.
Yield - Published: 75%
Actual: 35%
Advice: No residual product in picoline distillate or remaining on silica plug. Flask / reaction capable of withstanding increased pressure. Deep brown crude mixture, final product a green powder.
Spectra / Photos:
 |
| NMR, post-distillation |
 |
| TLC, from notebook |
Author Response: (When this post goes live, I'll send the link to the corresponding author. Any response will be published in due course)
**Thanks for reading our initial venture into collaborative "crowdsourced reaction validation." We appreciate any comments or suggestions for the next go-around. Want to get involved? Have an idea for another (cheap!) reaction to try? Talk to us in the Comments section!
